- +1 800 433 4609
- |
- Request Info
Sudomotor sympathetic response analysis
Introduction
Small nerve fibers are the first victims of diabetes, which affects the peripheral nervous system even if symptoms remain sub-clinical. According to a recent study, already at an early stage of diabetes, the sympathetic innervation of eccrine sweat glands density and function is progressively reduced.
SudoCheck has been created to enable a precise evaluation of sweat gland function. Based on a measuring method, patients place their hands and feet on stainless-steel electrodes. The method uses low voltage to stimulate the sweat glands and measure the electrochemical reaction between electrodes and chloride ions. This active, new method provides information and evidence of a sweat dysfunction that might otherwise not be detectable in physiological conditions. The measurement for the hands and feet express the quantitative results, while a risk score derives from demographic data and the measured values.
The SudoCheck system, in accordance with the 21 CFR 882.1540, represents a sympathetic skin response device that provides skin conductance measurements on a computer screen.
SudoCheck is, similar to a galvanic skin response stress test, a functional test that measures the sweat glands' capacity to release chloride ions following an electrochemical stimulation. It works by measuring the electrical potential difference caused by the electrochemical reaction of electrodes, which are applied to the skin and stimulated by a low voltage of variable amplitude. SudoCheck comes with 4x2 independent electrodes for placement on feet or palms and other areas with a high number of sweat glands.
SudoCheck provides information that determines the peripheral neuropathy and cardiometabolic risk in patients.
Advantages
Convenient for Patients: Requires no special preparation, fasting, or blood draws.
Rapid and Non-Invasive: Provides immediate, actionable results in just 3 minutes.
Designed for Clinical Versatility: Ideal for use by general practitioners, cardiologists, and neurologists to efficiently screen patients for cardiometabolic risk.
Essential in Diabetes Management: Accurately evaluates diabetic patients for autonomic neuropathy and associated neuropathic pain.
Clinically Relevant Findings: Results highlight the involvement of the peripheral autonomic nervous system in painful diabetic neuropathy.
Validated Accuracy: Results significantly correlate with clinical neuropathy evaluations, pain assessments, and autonomic function testing.
Clinical Utility of SudoCheck
The SudoCheck system is extensively proven and valuable in various clinical specialties, including:
Neurology
Effective in diagnosing and managing neuropathic pain, idiopathic neuropathies, diabetic neuropathy, and other small-fiber neuropathies often undetected by standard nerve conduction studies.
Cardiology And Internal Medicine
Essential for early detection of cardiometabolic risk related to autonomic dysfunction.
Pain Management
Early identification and ongoing monitoring of neuropathies, including complex pain disorders and Reflex Sympathetic Dystrophy (RSD).
Endocrinology
Early detection and monitoring of diabetic neuropathy, crucial for effective diabetic patient care.
Evaluation Of Sudomotor Function
Why evaluate sudomotor function?
The study of sudomotor presents a valuable tool to assess autonomic disorders. Sudomotor function is known to reflect sympathetic activity and provide insight into postganglionic autonomic innervation.
Recent clinical studies have shown that the level of glycemia influenced conventional tests. SudoCheck, however, has proven to be a reproducible and quantitative test method.
The need for a faster sudomotor function test
Sudomotor function assessment can now be carried out faster and simpler with the new SudoCheck technology.
In response to electrochemical activation, it measures the ability of sweat glands to release chloride ions and detects abnormalities. Once suspected or confirmed, more specialized tests are recommended to diagnose the underlying cause of the irregularity.
Evaluation of sudomotor function in diabetes
On of the significant causes of nerve damage is diabetes. Diabetic patients suffer from metabolic impairment and related inflammatory processes. As a result, their non-myelinated axons of small fiber nerves are primarily affected and known as Diabetic Autonomic Neuropathy (DAN).
There are presently several clinical studies ongoing to validate SudoCheck's new technology for DAN detection.
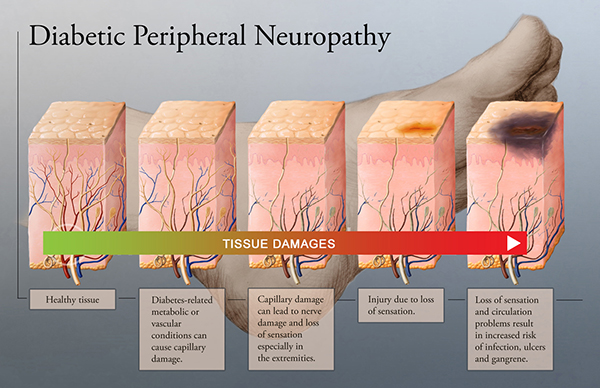
Small fiber nerves are early victims of diabetes
While symptoms remain sub-clinical, diabetes affects the peripheral nervous system, and small nerve fibers are usually the first victims. SudoCheck was developed as a new device to follow up on complications related to diabetes.
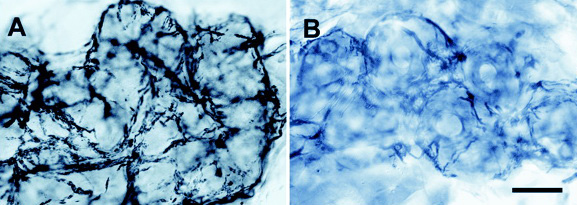
Left side - Normal innervation of a sweat gland at the distal leg in a healthy person.
Right side - Reduced innervation of a sweat gland in a diabetic patient.
Theoretical Background and Methods
The most prevalent complication of type-2 diabetes is Peripheral neuropathy. Cardiovascular Autonomic Neuropathy (CAN) has proven to be the highest risk factor for silent ischemia in patients with diabetes, and yet, in the field of neuropathy, it is probably still the most overlooked area. A non-biased and non-invasive way to assess sympathetic and parasympathetic modulation of the heart rate is HRV or Heart Rate Variability. It has largely been agreed upon that the parasympathetic system controls the High Frequency (HF) component, while the sympathetic system modulates the Low Frequency (LF) component. The accuracy of HRV analysis could be increased by exercise, as a joint consensus statement of the American Academy of Neurology the American Diabetes Association (ADA) confirms. Both bodies recommended that, to asses CAN, a series of Cardiovascular Autonomic Reflex Tests (CARTs) should be performed in assessing HRV during standing or deep breathing while monitoring postural systolic blood pressure fall, a method that is commonly referred to as the Ewing tests.
Sweat glands are stimulated by thin and non-myelinated sympathetic C-fibers that, depending on their lengths, can be damaged by peripheral neuropathy. With the understanding that functional disorder of sudomotor has been confirmed in pre-diabetes and diabetes, the ADA suggests in a consensus explanation that sudomotor function should be part of the diagnostic test for the early detection of neuropathies in diabetes patients. Despite the fact that a number of different methods have been developed, the lack of quick, easy-to-use tests for the diagnosis of sudomotor dysfunction has hindered its use in clinical practice in a broader format. With SudoCheck, a new device for quantitative assessment of sudomotor function has been developed. It is non-invasive, produces quick results and is even a useful tool to screen sympathetic system dysfunction in patients with Impaired Glucose Tolerance (IGT) or diabetes, as a number of studies have shown.
Eccrine sweat glands are early victims of diabetes
Small nerve fibers are the first victims of diabetes, which affects the peripheral nervous system even if symptoms remain sub-clinical. According to a recent study, already at an early stage of diabetes, the sympathetic innervation of eccrine sweat glands density and function is progressively reduced. Irrespective of temperature and physical exercise, this alteration of the sweat glands' autonomous control causes a long-lasting shift in the ionic balance of sweat conducts.
Reverse iontophoresis allows the measure of ionic balances in the sweat conducts
To detect ions, they have to be transported from the inside to the outside through the skin membrane. This process is called reverse iontophoresis. It entails an application of electrodes with a low voltage potential of variable amplitude to the skin, and observes the electrical potential difference caused by the electrochemical reaction on these electrodes. The SudoCheck device has 6 independent nickel electrodes that are placed on skin regions with a high density of sweat glands (palms, feet, forefront), and the data gathered provides a score that represents the individual's risk for show pre-diabetes (IGT), diabetes and complications. The system is automatically calibrated by a multiple electrode array, and after about two minutes of data collection, the results are displayed on a screen in graphical format.
In order to define the point of onset in regards to responses measured upon the use of low voltage potential with variable amplitudes to Ni electrodes, a study of the electrochemical behavior of nickel, in a set-up similar to that of the medical device, was carried out. The trial provided evidence, which clearly indicated that the main sweat parameter controlling the electrochemical currents is a result of the variation of chloride concentration.
In-vitro study and clinical observations were compared and evidently demonstrated the closeness of electrochemical in-vitro measurements on the behavior of nickel electrodes to those obtained through the clinical tests. It was further discovered that the determination of the current curve as a function of the potential (anode, cathode or their difference) supports a very efficient way to detect the deviation in Cl- concentration, at the level of the electrodes.
SudoCheck also analyzed the influence of chloride concentrations on the kinetics of the electrochemical reactions and to furnish a theoretical model, the proposed mechanisms and the obtained kinetic parameters were then used.
In this context, it could certainly be of interest to compare the electrochemical behavior and surface modification between electrodes aged in-vivo and electrodes aged in-vitro, as well as analyzing the electrochemical behavior of different compositions of stainless steel. Another interesting aspect could be the study of anodic reactions and their electrochemical kinetics when placed in physiological solutions at the surface of stainless steel electrodes.
